Picture source: Ben Aveling, Wikipedia, CC-BY-SA-4.0.
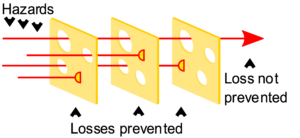
One of the common models of safety incidents is the Swiss Cheese Model, due to James Reason. Generally, there are multiple different layers of protection between you and exposure to a hazard, and no safety barrier is 100% protective. The model treats hazards as passing through one or more pores in these safety barriers—hence the “Swiss Cheese” name. If the holes in the layers of protection happen to line up, and a hazard challenges them, there is a safety incident.
Things to learn from the Swiss Cheese Model:
- No safety barrier is 100% effective.
- Using multiple safety barriers generally decreases the incidence of safety incidents.
- This is only true if the safety barriers are independent, that is, if the “holes” do not line up.
So when you look at a hazard in your lab, ask: How many independent safety barriers are protecting me? Then consider if you might need additional barriers.
If you have questions about layers of safety protection and the Swiss Cheese model, contact Dr. Daniel Kuespert, Homewood Laboratory Safety Advocate, at [email protected]. See Dr. Kuespert’s website, https://labsafety.jhu.edu, for more safety information. As always, emergency response is available from Security at 410-516-7777.
Daniel R. Kuespert, PhD, CSP | Laboratory Safety Advocate (he)
Johns Hopkins University
Krieger School of Arts & Sciences/Whiting School of Engineering
Ames Hall 241, Baltimore, MD 21218
P. 410-516-5525 | E. [email protected]
http://labsafety.jhu.edu